Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Last updated 19 novembro 2024

Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.
LA Weekend: Haunted Hayride; Jack-O'-Lantern Walk; Pull-A-Plane

Landon Loving on LinkedIn: Selecta, Sobi rout gout in pair of

Microfluidic Formulation of Topological Hydrogels for Microtissue
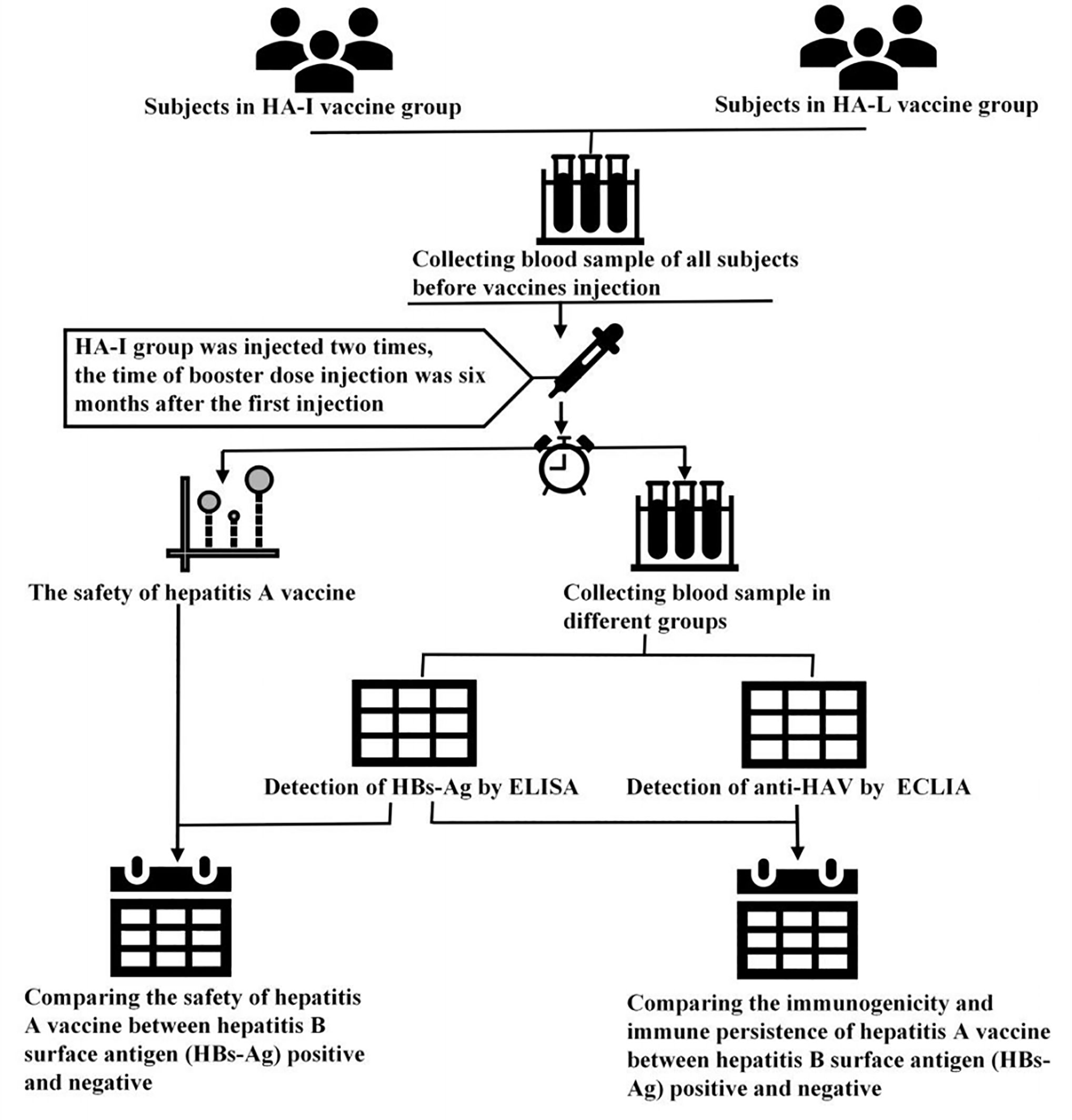
Frontiers The Safety, Immunogenicity, and Immunopersistence of

Materials about hepatitis B

Hepatitis B Foundation

IHEP (International Hepatology Education Program)

HBV replication inhibitors - ScienceDirect

Hepatitis B drug developers chart slow progress, just like in hep C

IHEP (International Hepatology Education Program)

Biotech Fierce Biotech
Recomendado para você
-
 some doors gifs : r/RobloxDoors19 novembro 2024
some doors gifs : r/RobloxDoors19 novembro 2024 -
 Robloxdoors Stories - Wattpad19 novembro 2024
Robloxdoors Stories - Wattpad19 novembro 2024 -
 TWO CRUCIFIX IN ONE ROOM?! (Doors Hotel+)#shorts #roblox #doors in 202319 novembro 2024
TWO CRUCIFIX IN ONE ROOM?! (Doors Hotel+)#shorts #roblox #doors in 202319 novembro 2024 -
 Paint Spray Booths: Construction, Types, Applications, and Benefits19 novembro 2024
Paint Spray Booths: Construction, Types, Applications, and Benefits19 novembro 2024 -
 Mrw someone door GIF - Find on GIFER19 novembro 2024
Mrw someone door GIF - Find on GIFER19 novembro 2024 -
 Roblox DOORS Speedrun 18:56 Solo on Make a GIF19 novembro 2024
Roblox DOORS Speedrun 18:56 Solo on Make a GIF19 novembro 2024 -
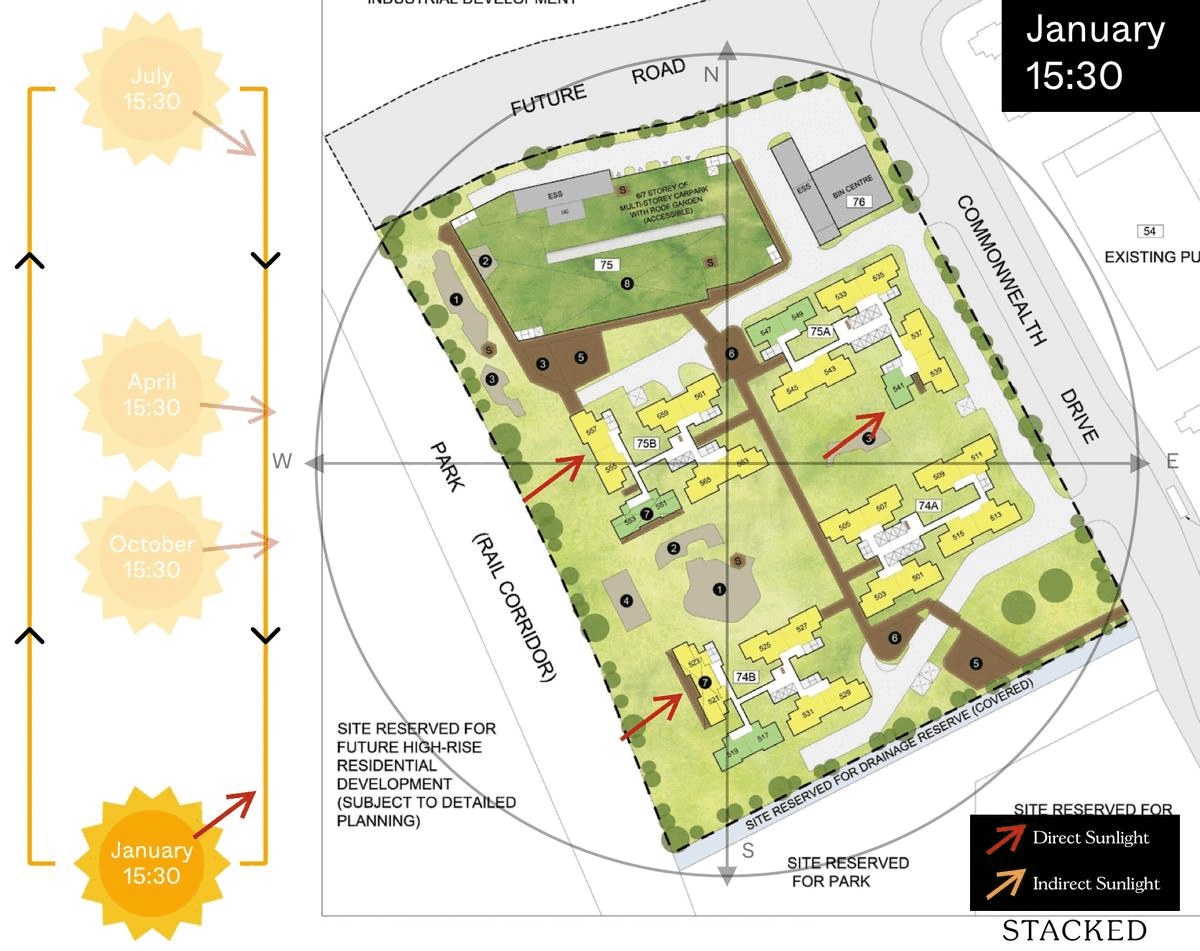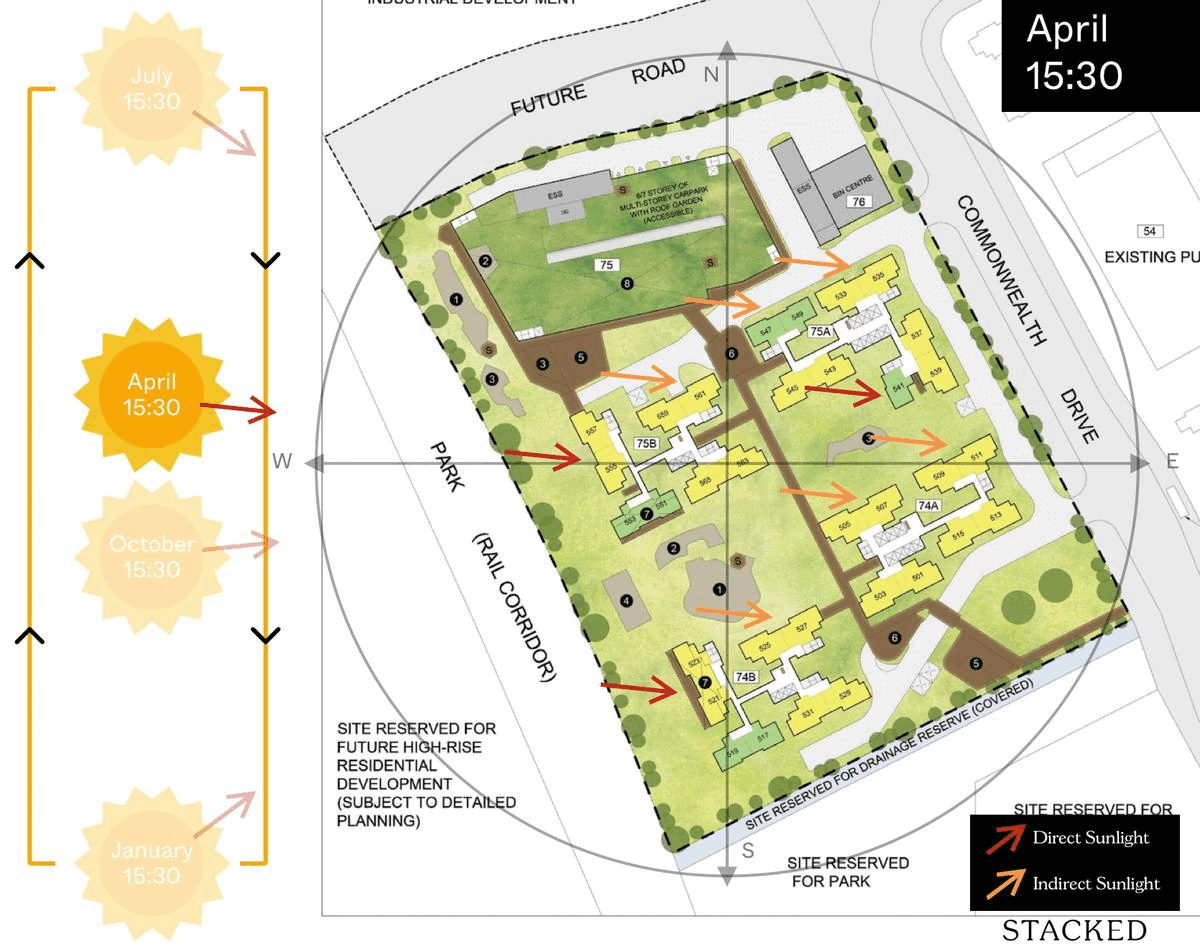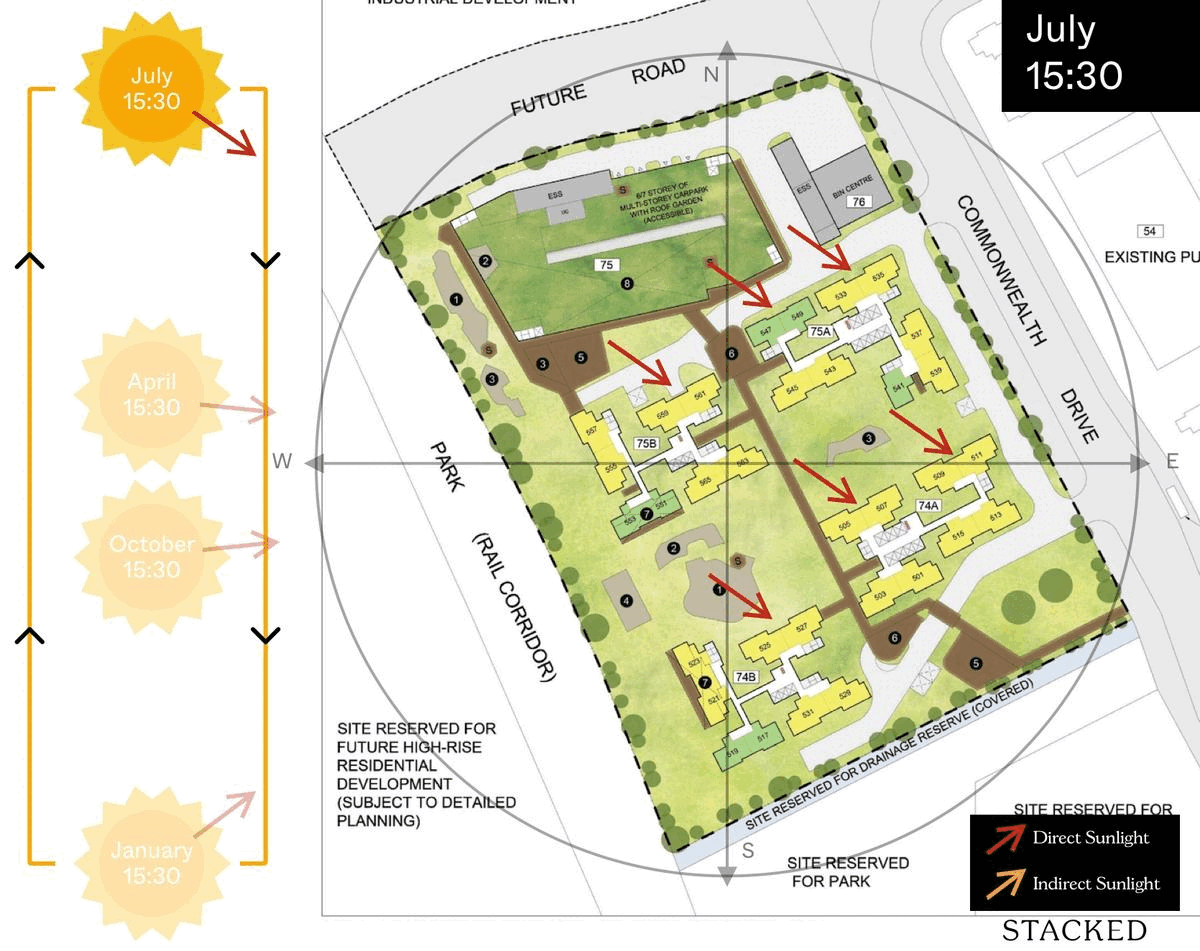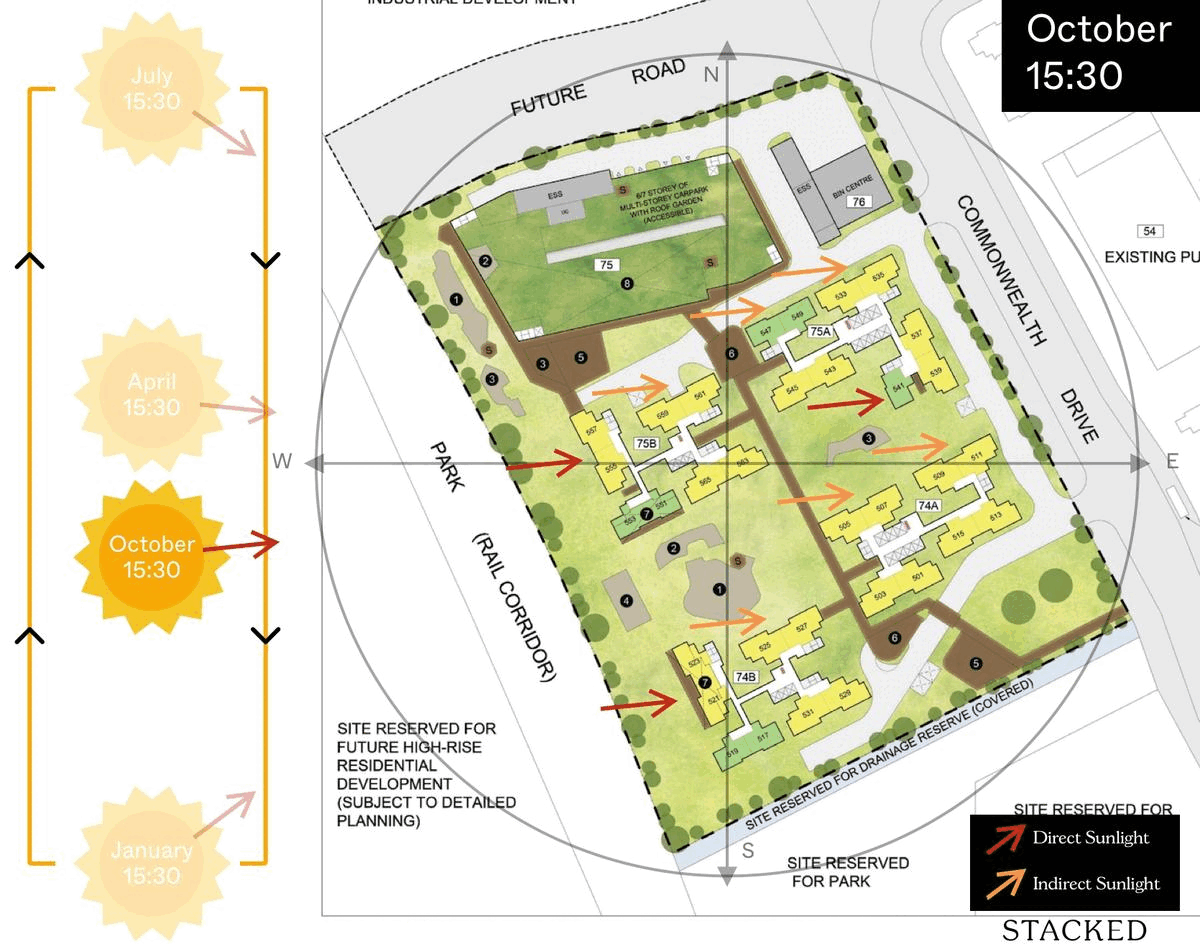 October 2023 BTO Launch Review: Ultimate Guide To Choosing The Best Unit19 novembro 2024
October 2023 BTO Launch Review: Ultimate Guide To Choosing The Best Unit19 novembro 2024 -
Knock at the Cabin' Review: M. Night Shyamalan's Anxious Masterpiece Is Best in Decades19 novembro 2024
-
 American Guns Drive the Migrant Crisis That Trump Wants to Fix With a Wall19 novembro 2024
American Guns Drive the Migrant Crisis That Trump Wants to Fix With a Wall19 novembro 2024 -
![Closed] Scripter! - Recruitment - Developer Forum](https://devforum-uploads.s3.dualstack.us-east-2.amazonaws.com/uploads/original/4X/d/b/9/db9ca5ad8c750c1d49743f66d1859c69fad9bef2.gif) Closed] Scripter! - Recruitment - Developer Forum19 novembro 2024
Closed] Scripter! - Recruitment - Developer Forum19 novembro 2024
você pode gostar
-
 Ellie's Tattoo : r/thelastofus19 novembro 2024
Ellie's Tattoo : r/thelastofus19 novembro 2024 -
 Movimentos das peças de Xadrez19 novembro 2024
Movimentos das peças de Xadrez19 novembro 2024 -
 Xbox one Stock Photos, Royalty Free Xbox one Images19 novembro 2024
Xbox one Stock Photos, Royalty Free Xbox one Images19 novembro 2024 -
 Tokonome Mamori, Valkyrie Drive Wiki19 novembro 2024
Tokonome Mamori, Valkyrie Drive Wiki19 novembro 2024 -
 Best Huohuo Build in Honkai: Star Rail19 novembro 2024
Best Huohuo Build in Honkai: Star Rail19 novembro 2024 -
 Doki Doki Literature Club Men's Play with Me T-Shirt Medium Black Natsuki Horror19 novembro 2024
Doki Doki Literature Club Men's Play with Me T-Shirt Medium Black Natsuki Horror19 novembro 2024 -
 Desenho Animado De Fogo Com Expressão De Choro E Boca Aberta Ilustração do Vetor - Ilustração de divertimento, liso: 25447547619 novembro 2024
Desenho Animado De Fogo Com Expressão De Choro E Boca Aberta Ilustração do Vetor - Ilustração de divertimento, liso: 25447547619 novembro 2024 -
 Lendário Rn7 Significa Rota Nacional Que Atravessa Savana Selvagem Vermelha Africana Com Pequenas árvores E Arbustos Nos Lados Imagem de Stock - Imagem de paisagem, destino: 17423289919 novembro 2024
Lendário Rn7 Significa Rota Nacional Que Atravessa Savana Selvagem Vermelha Africana Com Pequenas árvores E Arbustos Nos Lados Imagem de Stock - Imagem de paisagem, destino: 17423289919 novembro 2024 -
 KSI Jake Paul : KSI vs IShowSpeed during Jake Paul's boxing match: Check date, key details19 novembro 2024
KSI Jake Paul : KSI vs IShowSpeed during Jake Paul's boxing match: Check date, key details19 novembro 2024 -
![KOF 97 Online] Factory - [ HIGH RES ROOM ] - Mugen Free For All](https://i.imgur.com/VhkUUdq.png) KOF 97 Online] Factory - [ HIGH RES ROOM ] - Mugen Free For All19 novembro 2024
KOF 97 Online] Factory - [ HIGH RES ROOM ] - Mugen Free For All19 novembro 2024