What You Should Know About CSV in Pharma
Por um escritor misterioso
Last updated 16 novembro 2024

Learn more about computer system validation, which is required by the FDA and other global regulatory bodies for drug and medical device manufacturers.
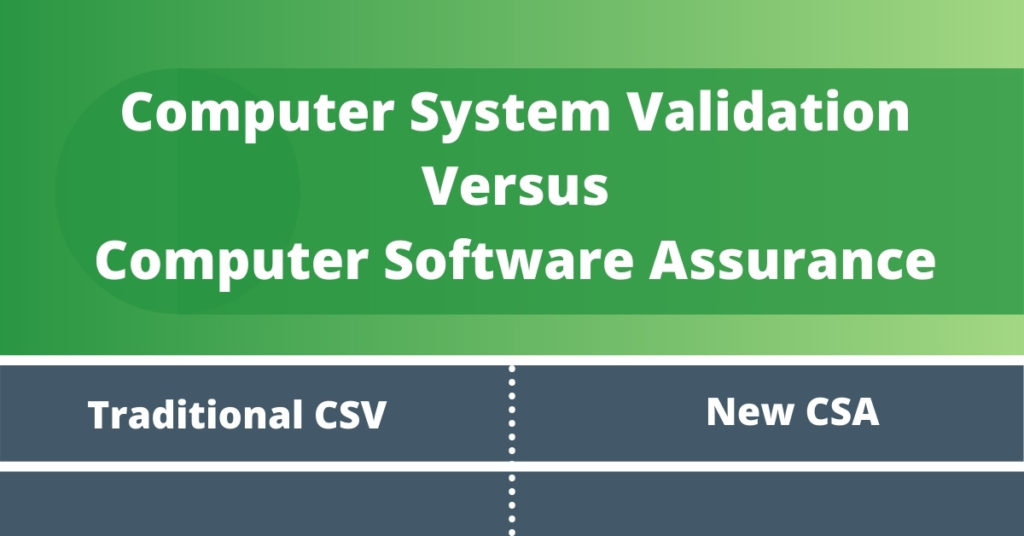
Infographic: Computer System Validation Vs. Computer Software Assurance - SL Controls

What is Computer System Validation and How Do You Do It?
PDF) Computerized Systems Validation (CSV) in Biopharmaceutical Industries
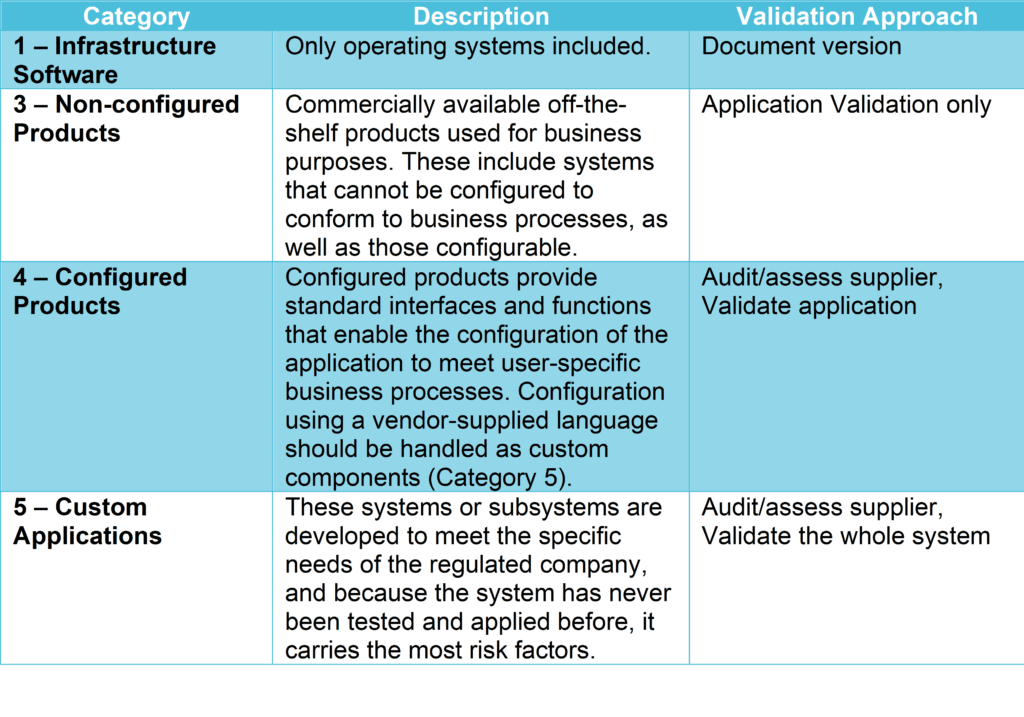
Risk-Based Computerized System Validation (CSV) and Computer Software Assurance (CSA) - Old Wine in a New Bottle? - Kvalito
CSV is a Profession Too

Why is Computer System Validation so important? - Express Pharma
Preparing for the GAMP Transition to Computer Software Assur
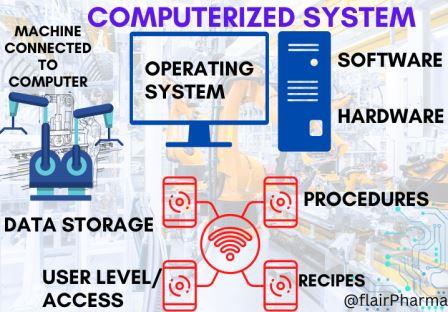
Computer System Validation (CSV) In Pharmaceuticals (23) » Flairpharma

Pharmaceutical Computer System Validation - CSV Validation in Pharma
Recomendado para você
-
Escola Deuzuita de Queiroz vai participar de Feira de Tecnologia16 novembro 2024
-
 DataMap PO SmartView Coupa App Marketplace16 novembro 2024
DataMap PO SmartView Coupa App Marketplace16 novembro 2024 -
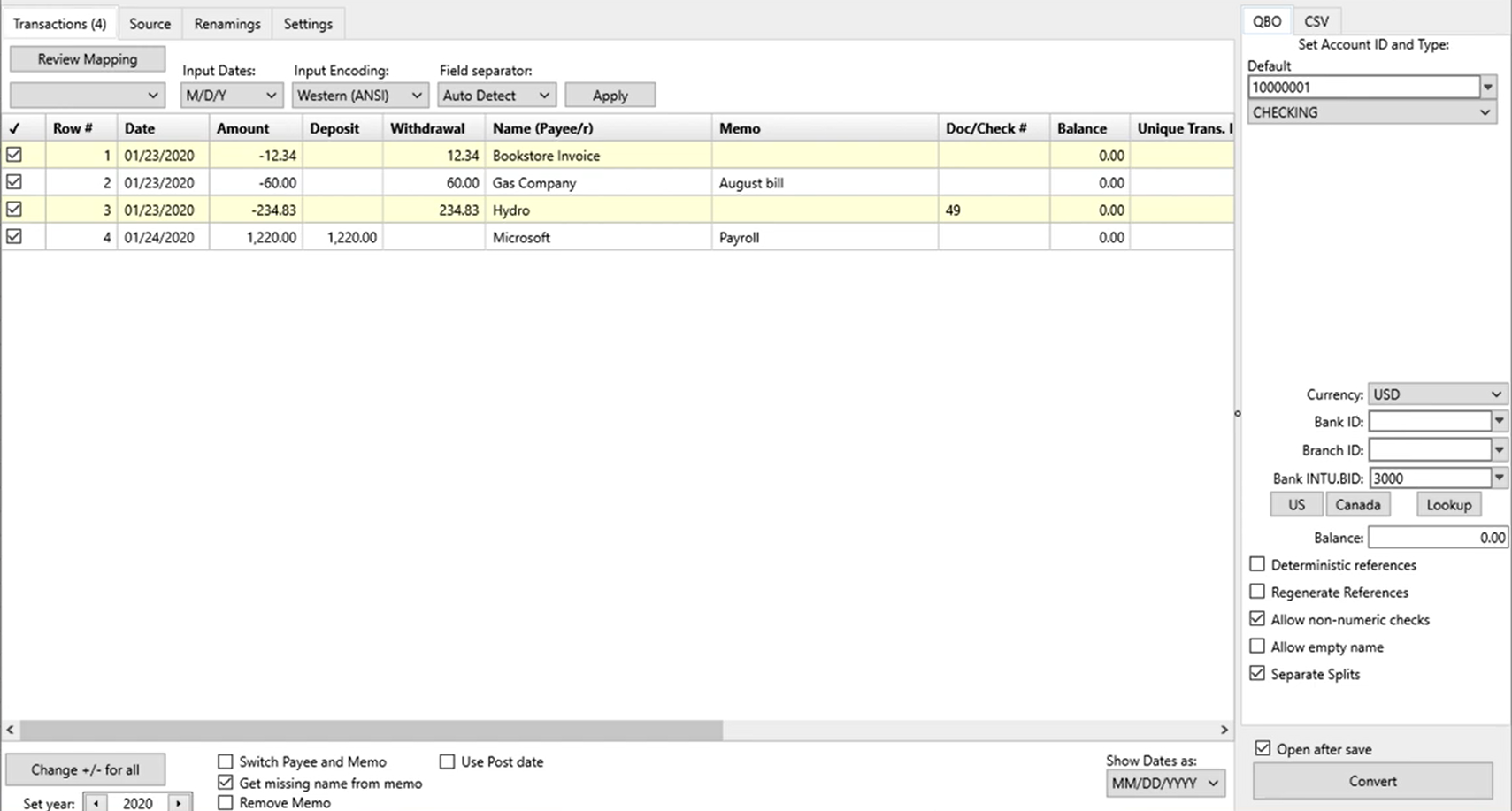 How To Map CSV files16 novembro 2024
How To Map CSV files16 novembro 2024 -
 CRVPM Level VI/Certified AICPA SOC® Report Analyst (CASRA16 novembro 2024
CRVPM Level VI/Certified AICPA SOC® Report Analyst (CASRA16 novembro 2024 -
 IBM Computerized System Validation CSV16 novembro 2024
IBM Computerized System Validation CSV16 novembro 2024 -
 Merging Multiple CSV Files Using Pandas, by Prashant Gaur16 novembro 2024
Merging Multiple CSV Files Using Pandas, by Prashant Gaur16 novembro 2024 -
Solved: Simple CSV comma Issue, I think - Power Platform Community16 novembro 2024
-
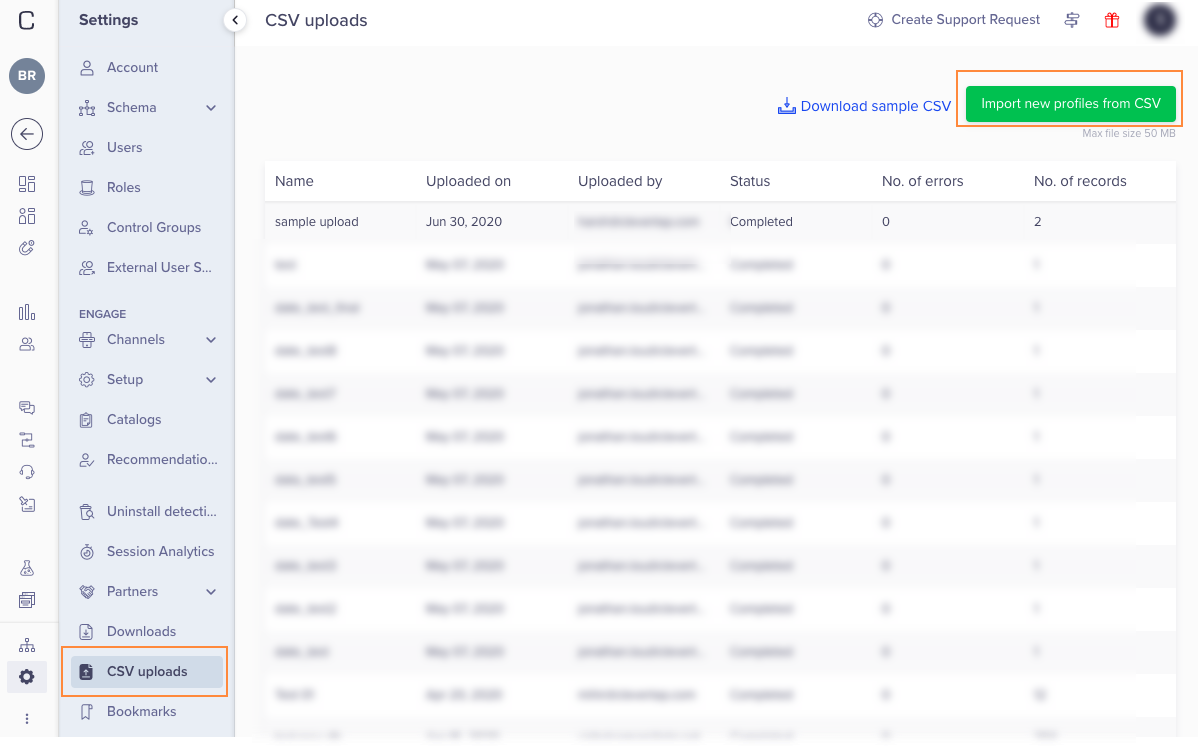 CSV Upload16 novembro 2024
CSV Upload16 novembro 2024 -
 2012 Audi A4 2.0 TDI S LINE 2.0 Diesel Manual - £8500 - PMA Cars16 novembro 2024
2012 Audi A4 2.0 TDI S LINE 2.0 Diesel Manual - £8500 - PMA Cars16 novembro 2024 -
 Computer System Validation (CSV) to Computer Software Assurance (CSA): Taking a More Risked-Based Approach - Verista16 novembro 2024
Computer System Validation (CSV) to Computer Software Assurance (CSA): Taking a More Risked-Based Approach - Verista16 novembro 2024
você pode gostar
-
 História da Equipe Rocket chega ao fim na série animada de Pokémon16 novembro 2024
História da Equipe Rocket chega ao fim na série animada de Pokémon16 novembro 2024 -
 animegame 5 image - Dragon Ball Z Online - IndieDB16 novembro 2024
animegame 5 image - Dragon Ball Z Online - IndieDB16 novembro 2024 -
 Microsoft Xbox Series S 512GB Game All-Digital Console + 1 Xbox Wireless1 Controller, White - 1440p Gaming Resolution, 4K Streaming Media Playback, WiFi (Renewed) : Video Games16 novembro 2024
Microsoft Xbox Series S 512GB Game All-Digital Console + 1 Xbox Wireless1 Controller, White - 1440p Gaming Resolution, 4K Streaming Media Playback, WiFi (Renewed) : Video Games16 novembro 2024 -
Fortza Hermannstadt16 novembro 2024
-
 Summer Walker, Bryson Tiller - Playing Games ( Lyrics )16 novembro 2024
Summer Walker, Bryson Tiller - Playing Games ( Lyrics )16 novembro 2024 -
 Agora é possível jogar a versão original de Tetris pelo navegador16 novembro 2024
Agora é possível jogar a versão original de Tetris pelo navegador16 novembro 2024 -
 Cheat Engine v7.3 APK Download For Android16 novembro 2024
Cheat Engine v7.3 APK Download For Android16 novembro 2024 -
 Oshi no Ko Kana Arima Look Up Series figure, MegaHouse16 novembro 2024
Oshi no Ko Kana Arima Look Up Series figure, MegaHouse16 novembro 2024 -
 Marvel Movies16 novembro 2024
Marvel Movies16 novembro 2024 -
 Avaliando aplicativos de Xadrez desconhecidos.16 novembro 2024
Avaliando aplicativos de Xadrez desconhecidos.16 novembro 2024

