What will be the shape of ICl 2 among the following?A. BentB. Trigonal planarC. LinearD. Trigonal bipyramidal
Por um escritor misterioso
Last updated 15 novembro 2024

What will be the shape of ICl 2 among the following?A. BentB. Trigonal planarC. LinearD. Trigonal bipyramidal
Geometry of Molecules - Chemistry LibreTexts

What is the molecular geometry of ICl_3?
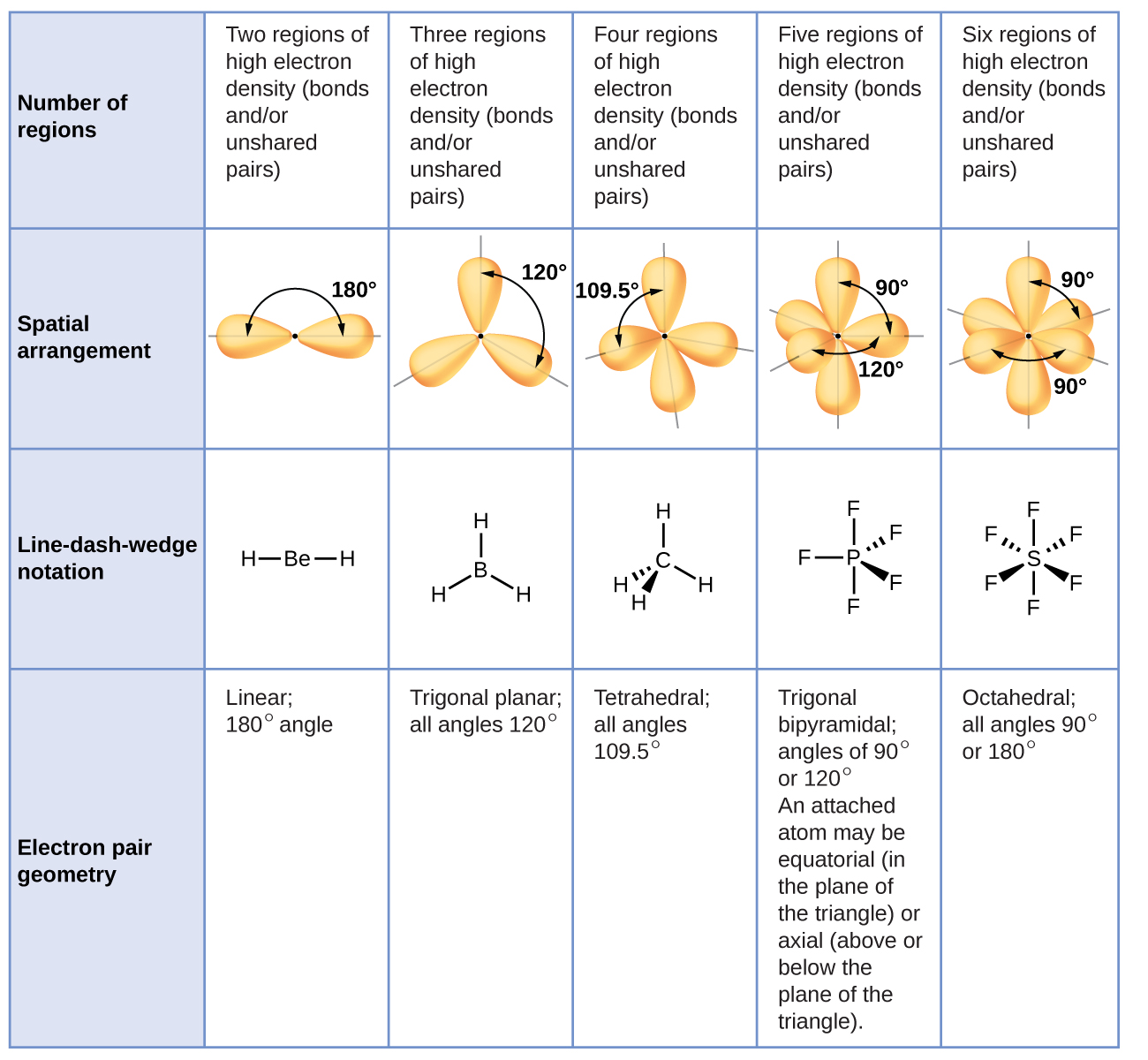
3.3 Molecular Structure and Polarity – Chemical Bonding and

Calaméo - Test Bank For Chemistry A Molecular Approach 2nd Edition
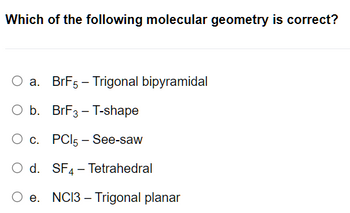
Answered: Which of the following molecular…

Use the VSEPR theory to predict the shape of carbon tetrachloride
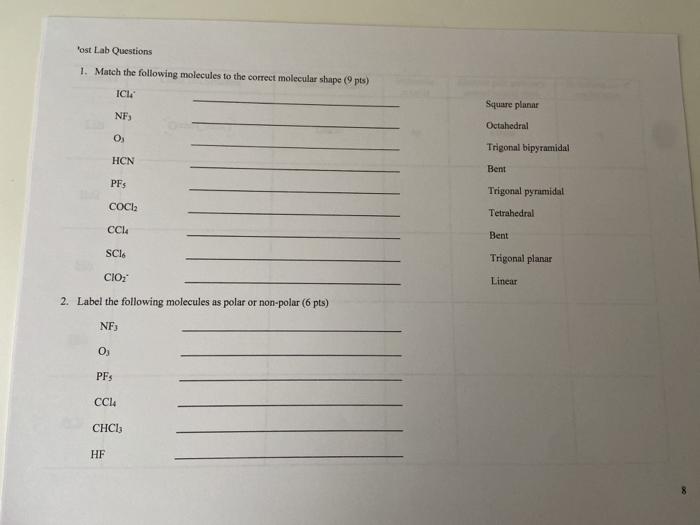
Solved 'ost Lab Questions 1. Match the following molecules
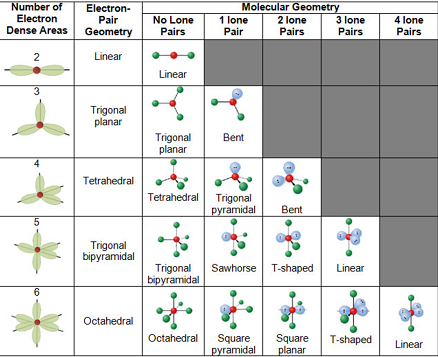
VSEPR Theory - Postulates, Limitations, Predicting Shapes
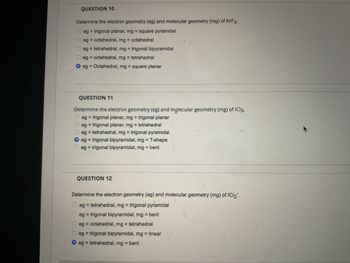
Answered: QUESTION 10 Determine the electron…
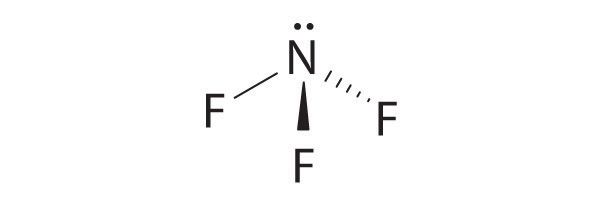
VSEPR
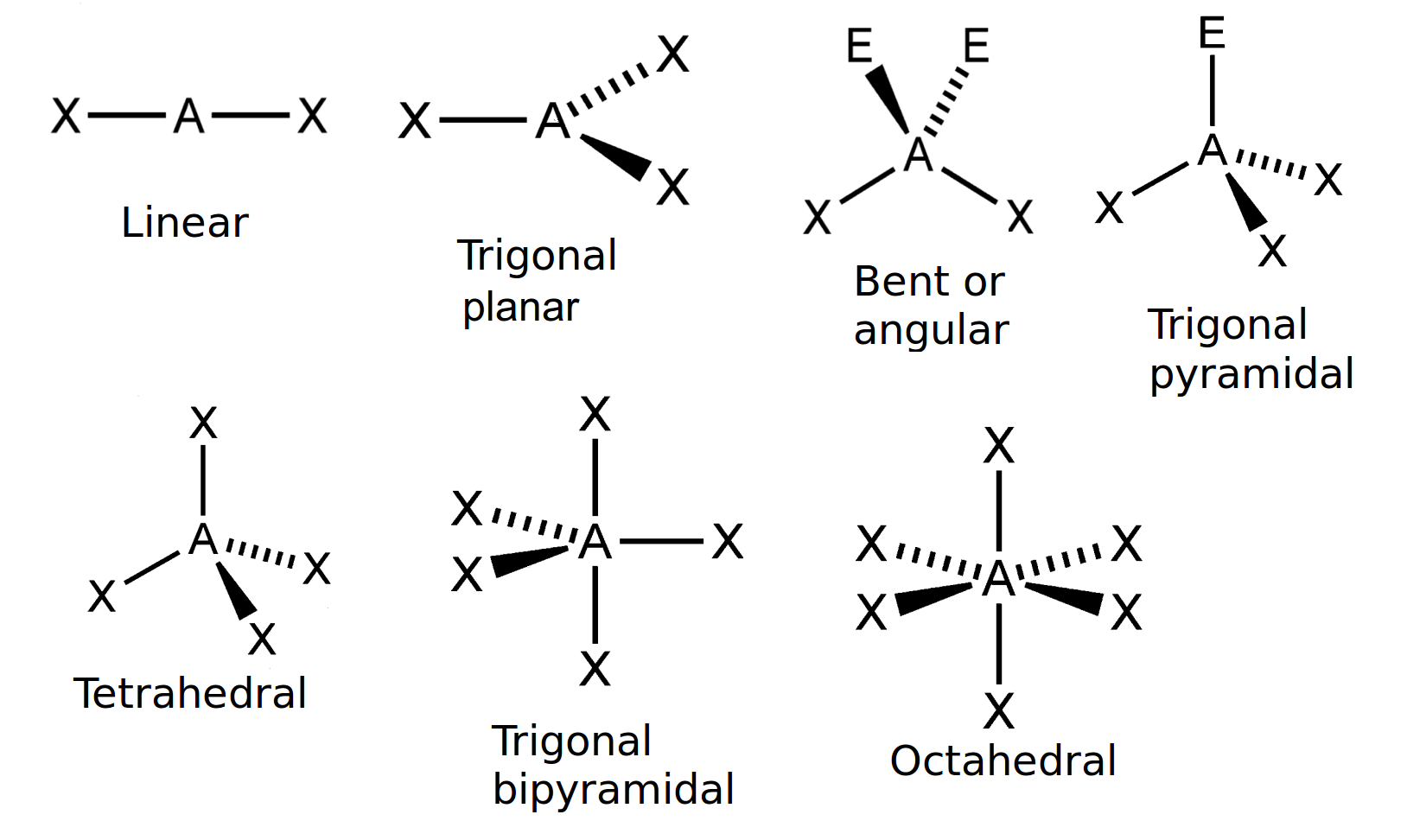
3.2 Molecular shape, Atomic combinations

What is the hybridization of Xe in XeOFs and what is the molecular
Recomendado para você
-
Royal Aircraft Factory B.E.2 - Wikipedia15 novembro 2024
-
 G-AWYI - Private Royal Aircraft Factory BE.2 at Brighton - Shoreham, Photo ID 64915915 novembro 2024
G-AWYI - Private Royal Aircraft Factory BE.2 at Brighton - Shoreham, Photo ID 64915915 novembro 2024 -
 File:Royal Aircraft Factory B.E.2c '2699' (14850845263).jpg - Wikimedia Commons15 novembro 2024
File:Royal Aircraft Factory B.E.2c '2699' (14850845263).jpg - Wikimedia Commons15 novembro 2024 -
 Tênis adidas DNA x LEGO® Two-Strap - Preto adidas | adidas Brasil15 novembro 2024
Tênis adidas DNA x LEGO® Two-Strap - Preto adidas | adidas Brasil15 novembro 2024 -
 9.5 Molecular Orbital Theory - Chad's Prep®15 novembro 2024
9.5 Molecular Orbital Theory - Chad's Prep®15 novembro 2024 -
2 Be 3 - song and lyrics by 2 Be 315 novembro 2024
-
 12 best Hoka shoes for running and walking in 202315 novembro 2024
12 best Hoka shoes for running and walking in 202315 novembro 2024 -
 Anthropic \ Claude 215 novembro 2024
Anthropic \ Claude 215 novembro 2024 -
 How to draw the Be2+ Lewis Dot Structure.15 novembro 2024
How to draw the Be2+ Lewis Dot Structure.15 novembro 2024 -
 Tenis Vans Kyle Pro 2 Black/White - Lobster - Be a Lobstar - Tênis, Roupas, Acessórios e Mais15 novembro 2024
Tenis Vans Kyle Pro 2 Black/White - Lobster - Be a Lobstar - Tênis, Roupas, Acessórios e Mais15 novembro 2024
você pode gostar
-
 FINALMENTE👀CRIEI MEU SCRIPT PARA BLOX FRUITS🍎SEM KEY E SEM RESETAR A CONTA!PEGA TUDO⚡MOBILE E PC📱💻15 novembro 2024
FINALMENTE👀CRIEI MEU SCRIPT PARA BLOX FRUITS🍎SEM KEY E SEM RESETAR A CONTA!PEGA TUDO⚡MOBILE E PC📱💻15 novembro 2024 -
 How to Fix 'Unable to Authenticate' Error on Pokemon Go?15 novembro 2024
How to Fix 'Unable to Authenticate' Error on Pokemon Go?15 novembro 2024 -
 Edit roblox Cute tumblr wallpaper, Roblox animation, Roblox pictures15 novembro 2024
Edit roblox Cute tumblr wallpaper, Roblox animation, Roblox pictures15 novembro 2024 -
 Piscina Clássica 3D Bola 8 versão móvel andróide iOS apk baixar gratuitamente-TapTap15 novembro 2024
Piscina Clássica 3D Bola 8 versão móvel andróide iOS apk baixar gratuitamente-TapTap15 novembro 2024 -
 SCP Foundation SCP – Containment Breach Gay LGBT community, scp15 novembro 2024
SCP Foundation SCP – Containment Breach Gay LGBT community, scp15 novembro 2024 -
![Detroit Become Human Realistic Ultra Graphics Gameplay [4K UHD 60FPS] Full Game](https://i.ytimg.com/vi/8ORsaslz1bk/maxresdefault.jpg) Detroit Become Human Realistic Ultra Graphics Gameplay [4K UHD 60FPS] Full Game15 novembro 2024
Detroit Become Human Realistic Ultra Graphics Gameplay [4K UHD 60FPS] Full Game15 novembro 2024 -
 Spoiler Blue Lock chap 237: Chiến thắng đã được định đoạt!15 novembro 2024
Spoiler Blue Lock chap 237: Chiến thắng đã được định đoạt!15 novembro 2024 -
 yo en kaguya-sama: love is war - cap 1 - Wattpad15 novembro 2024
yo en kaguya-sama: love is war - cap 1 - Wattpad15 novembro 2024 -
 Roblox CEO says metaverse is still huge opportunity15 novembro 2024
Roblox CEO says metaverse is still huge opportunity15 novembro 2024 -
 Boneca Bebê Reborn 100% Silicone 23 Itens Bolsa Maternidade15 novembro 2024
Boneca Bebê Reborn 100% Silicone 23 Itens Bolsa Maternidade15 novembro 2024

