ANANDA Scientific Announces FDA approval of the IND for the Clinical Trial on the Treatment of Opioid Use Disorder (OUD)
Por um escritor misterioso
Last updated 20 novembro 2024

ANANDA Scientific Inc., (a biotech pharma company) today announced approval by the U.S. Food and Drug Administration (FDA) of the Investigational New

The Opioid Crisis and Recent Federal Policy Responses

TESCREAL hallucinations: Psychedelic and AI hype as inequality engines in: Journal of Psychedelic StudiesOnline First
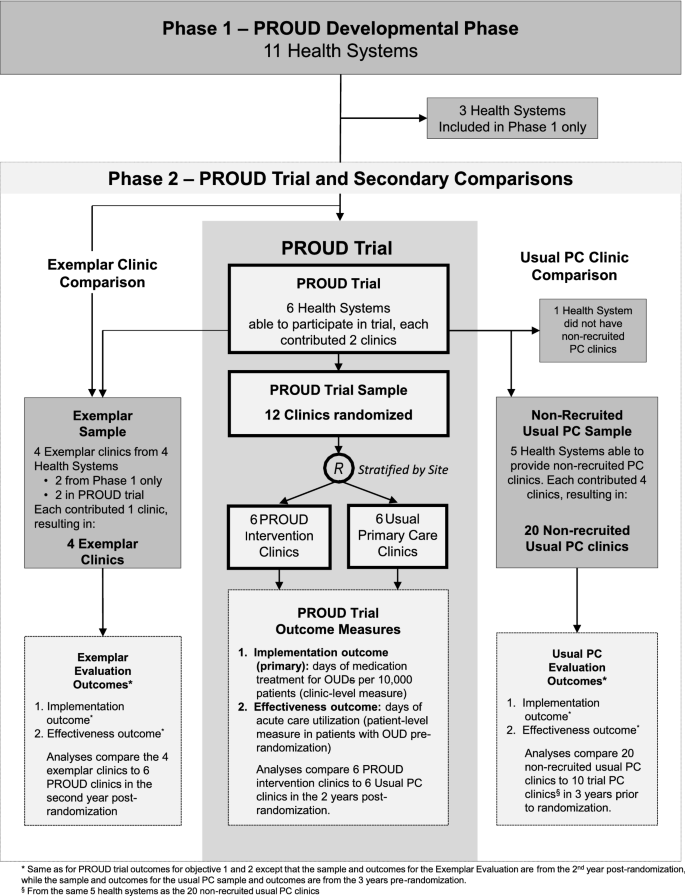
PRimary Care Opioid Use Disorders treatment (PROUD) trial protocol: a pragmatic, cluster-randomized implementation trial in primary care for opioid use disorder treatment, Addiction Science & Clinical Practice

The Opioid Crisis and Recent Federal Policy Responses

g556911.jpg

Substance Abuse Treatment Market Size & Share Analysis - Industry Research Report - Growth Trends

Public Comment Index for the National Alzheimer's Project Act

Ananda Scientific (@AnandaScience) / X

Ananda Scientific (@AnandaScience) / X

MUSC Catalyst News, MUSC

Substance Abuse Treatment Market Size & Share Analysis - Industry Research Report - Growth Trends

ANANDA Scientific Announces FDA approval of the IND for the Clinical Trial on the Treatment of Opioid Use Disorder (OUD)
Recomendado para você
-
 Pegou todo mundo de surpresa, diz Ananda, cantora do hit Quero Que Tu Vá - Revista Marie Claire20 novembro 2024
Pegou todo mundo de surpresa, diz Ananda, cantora do hit Quero Que Tu Vá - Revista Marie Claire20 novembro 2024 -
 Ananda cosmetic20 novembro 2024
Ananda cosmetic20 novembro 2024 -
:max_bytes(150000):strip_icc()/ananda-eidelstein-headshot-39e5124d51f249279f0c111acbc59c26.jpg) Ananda Eidelstein - Real Simple20 novembro 2024
Ananda Eidelstein - Real Simple20 novembro 2024 -
 Ayurveda, Counselling, Reiki, Yoga & Meditation20 novembro 2024
Ayurveda, Counselling, Reiki, Yoga & Meditation20 novembro 2024 -
Ananda - Indian Restaurant, Vegetarian, Kosher20 novembro 2024
-
 ACT 4 SA's Ananda Tomas leads the fight for police reform in San Antonio20 novembro 2024
ACT 4 SA's Ananda Tomas leads the fight for police reform in San Antonio20 novembro 2024 -
 Ananda Los Angeles20 novembro 2024
Ananda Los Angeles20 novembro 2024 -
 Ananda Meditation App Guided Meditations and Techniques for iOS and Android — Ananda20 novembro 2024
Ananda Meditation App Guided Meditations and Techniques for iOS and Android — Ananda20 novembro 2024 -
 Trijunto Ananda Caramelo20 novembro 2024
Trijunto Ananda Caramelo20 novembro 2024 -
 Ananda Morais Cabelo, Roupas20 novembro 2024
Ananda Morais Cabelo, Roupas20 novembro 2024
você pode gostar
-
 Samsung Galaxy S23 Plus Price in Malaysia & Specs - RM365820 novembro 2024
Samsung Galaxy S23 Plus Price in Malaysia & Specs - RM365820 novembro 2024 -
Estruturas de Madeira (Aula 3), PDF, Madeira20 novembro 2024
-
 Forum Barreiro garante maioria da sua area bruta locavel Oferta comercial reforcada em oito loja - Rostos On-line20 novembro 2024
Forum Barreiro garante maioria da sua area bruta locavel Oferta comercial reforcada em oito loja - Rostos On-line20 novembro 2024 -
 Top Twitch streamers: the Twitch streamers with the most followers - Video Games on Sports Illustrated20 novembro 2024
Top Twitch streamers: the Twitch streamers with the most followers - Video Games on Sports Illustrated20 novembro 2024 -
 Quiz: futebol europeu #futebol #quiz #igeocraft20 novembro 2024
Quiz: futebol europeu #futebol #quiz #igeocraft20 novembro 2024 -
Ninja Donghua - Apotheosis Capítulos 272.1, 272.2 e 272.320 novembro 2024
-
![Ravena - Todas as cenas de Poderes & Lutas #2 [Os Jovens Titãs]](https://i.ytimg.com/vi/mfTiVi_vTY4/maxresdefault.jpg) Ravena - Todas as cenas de Poderes & Lutas #2 [Os Jovens Titãs]20 novembro 2024
Ravena - Todas as cenas de Poderes & Lutas #2 [Os Jovens Titãs]20 novembro 2024 -
 Quero jogar futebol: menina abusada pode encontrar acolhimento no esporte - UOL Universa20 novembro 2024
Quero jogar futebol: menina abusada pode encontrar acolhimento no esporte - UOL Universa20 novembro 2024 -
 Review - Snoke's Throne Room In Marvel's Star Wars: The Last Jedi #5 - Star Wars News Net20 novembro 2024
Review - Snoke's Throne Room In Marvel's Star Wars: The Last Jedi #5 - Star Wars News Net20 novembro 2024 -
 Alien World-Rick and Morty Live Wallpaper20 novembro 2024
Alien World-Rick and Morty Live Wallpaper20 novembro 2024


